Abstract
During hematopoiesis there is a balance between divergent functions such as self-renewal, differentiation, and proliferation. This process allows for continued blood formation throughout life as well as hematopoietic regeneration after periods of stress. It was recently shown that suppression of sex steroids, in addition to promoting T and B cell lymphopoiesis, promotes hematopoietic rejuvenation by mediating functional changes to HSC pool (Khong et al., 2015).
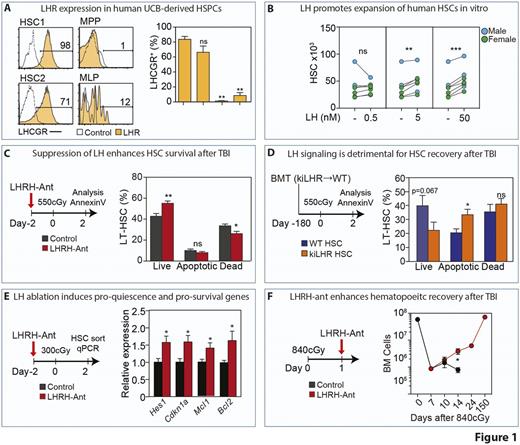
Although those changes suggested a direct impact of testosterone on HSC function, when we examined the expression of hormone receptors on the hematopoietic compartment, we found almost no detectable expression of the androgen receptor. In contrast, expression of luteinizing hormone (LHR) was highly enriched on HSCs with nearly absent expression in downstream hematopoietic progenitor cells as well as in the stromal cell compartment. Consistent with the mouse data, high expression of LHR was also found in human HSCs (Fig. 1A). Given the peculiar expression of the LHR on HSCs, we sought to examine the direct effect of LH on HSC function. LH significantly enhanced mouse HSC colony formation in cobblestone area-forming cell and colony-forming cell assays. In addition, LH significantly expand human HSCs in a stroma-free culture system, without impacting on their colony-forming potential (Fig. 1B).
Given the direct effects of LH on HSCs, we investigated if blocking LH could attenuate HSC proliferation in two different models of hematopoietic stress that force HSCs out of their quiescent status. Pharmacological suppression of LH using a luteinizing hormone-releasing hormone-antagonist (LHRH-Ant) inhibits HSCs from entry into cell cycle after Poly I:C and sub-lethal dose of total body irradiation (SL-TBI) exposure. Importantly, LH inhibition also significantly improved the proportion of live HSCs after SL-TBI (Fig. 1C). To gain some insights into the direct impact of LH on HSCs, we generated a hematopoietic chimera using KiLHRD582G mice, which have a constitutively-active D582G mutation in the Lhr gene. While there was no difference in primary reconstitution in mice receiving WT or KiLHRD582G HSCs, we found that mice reconstituted with KiLHRD582G and subsequently exposed to SL-TBI showed significantly higher proportion of apoptotic HSC compared to mice reconstituted with WT HSCs (Fig. 1D). To elucidate the molecular changes induced by LH inhibition, we assessed the expression of genes associated with quiescence, proliferation, DNA damage and apoptosis in HSC after SL-TBI. We found that LH suppression promoted higher levels of Hes1, Cdkn1a (p21), Bcl2 and Mcl1, genes previously identified to enforce HSC quiescence and survival (Fig. 1E). Consistent with these data, mice treated with the LHRH-Ant showed improved survival when exposed to successive doses of 5-fluorouracil, a well-established method to challenge HSC quiescence and self-renewal potential. It has been previously showing that promoting quiescence early after hematopoietic insults can mitigate HSC exhaustion and, ultimately, promote hematopoietic recovery and mouse survival. Mice receiving LHRH-Ant 24h after lethal TBI (840cGy) exposure showed a significant increase in survival compared to control animals. In fact, LH suppression spared LT-HSCs from radiation toxicity thus promoting hematopoietic recovery (Fig. 1F). Consistent with a negative role of LH on HSC recovery after radiation, mice deficient for LHR had a statistically significant increase in survival when exposed to L-TBI compared to control littermate.
In conclusion our studies demonstrate that LH can directly regulate HSC quiescence, and that LH inhibition with a LHRH-Ant can be used as a radiation mitigation strategy.
Jenq: Seres: Research Funding. Kiem: Rocket Pharmaceuticals: Consultancy, Equity Ownership, Patents & Royalties, Research Funding. van den Brink: Seres: Research Funding; Jazz Pharmaceuticals: Consultancy; Therakos Institute: Other: Speaking engagement; PureTech Health: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.